Article: Crowe, R. P., Myers, J. B., Fernandez, A. R., Bourn, S., & McMullan, J. T. (2020). The cincinnati prehospital stroke scale compared to stroke severity tools for large vessel occlusion stroke prediction. Prehospital Emergency Care, 1-9.
Background: Since the first trials demonstrating significant benefit from endovascular therapy for patients with stroke secondary to large vessel occlusion (LVO) were published, the EMS community has been actively refining screening and destination protocols to optimize patient outcomes through destination choice. Indeed, given a ~ 10% decrease in good functional outcome for every 30 minutes in delay in endovascular treatment, appropriate field triage is a critical component of stroke systems of care for patients with an LVO. [1,2]
LVO Screening tools of variable complexity have been developed. Depending on the complexity, implementation of such screening tools requires significant investment in training, documentation and tracking on the part of the EMS system. In general, the sensitivity, specificity and positive predictive value of all the LVO scales has been suboptimal. [3] In contrast, the Cincinnati Prehospital Stroke Scale (CPSS) is widely implemented and regularly performed by EMS clinicians at all scopes of practice. The objective of this paper was to address whether newly developed LVO scales offer a clinically-meaningful advantage over the CPSS.
Methods: This study was a retrospective analysis of prehospital electronic care records with linked hospital outcome data for the 2018 calendar year from the ESO research database. Inclusion criteria included 911 responses with one or more of the stroke scales of interest (CPSS, RACE, LAMS, or VAN) documented AND linked hospital diagnosis data available. Patients were classified as having intracerebral hemorrhage, transient ischemic attack or acute ischemic stroke based the hospital ICD-10 code. If the ICD-10 code indicated thrombosis or embolism of the middle cerebral arteries, internal carotid artery or basilar artery, the patient was categorized as having an LVO. Sensitivity and specificity for detection of LVO for each documented stroke scale was calculated. Receiver Operating curves were used to assess the overall discrimination of each scale for LVO.
Key Results:
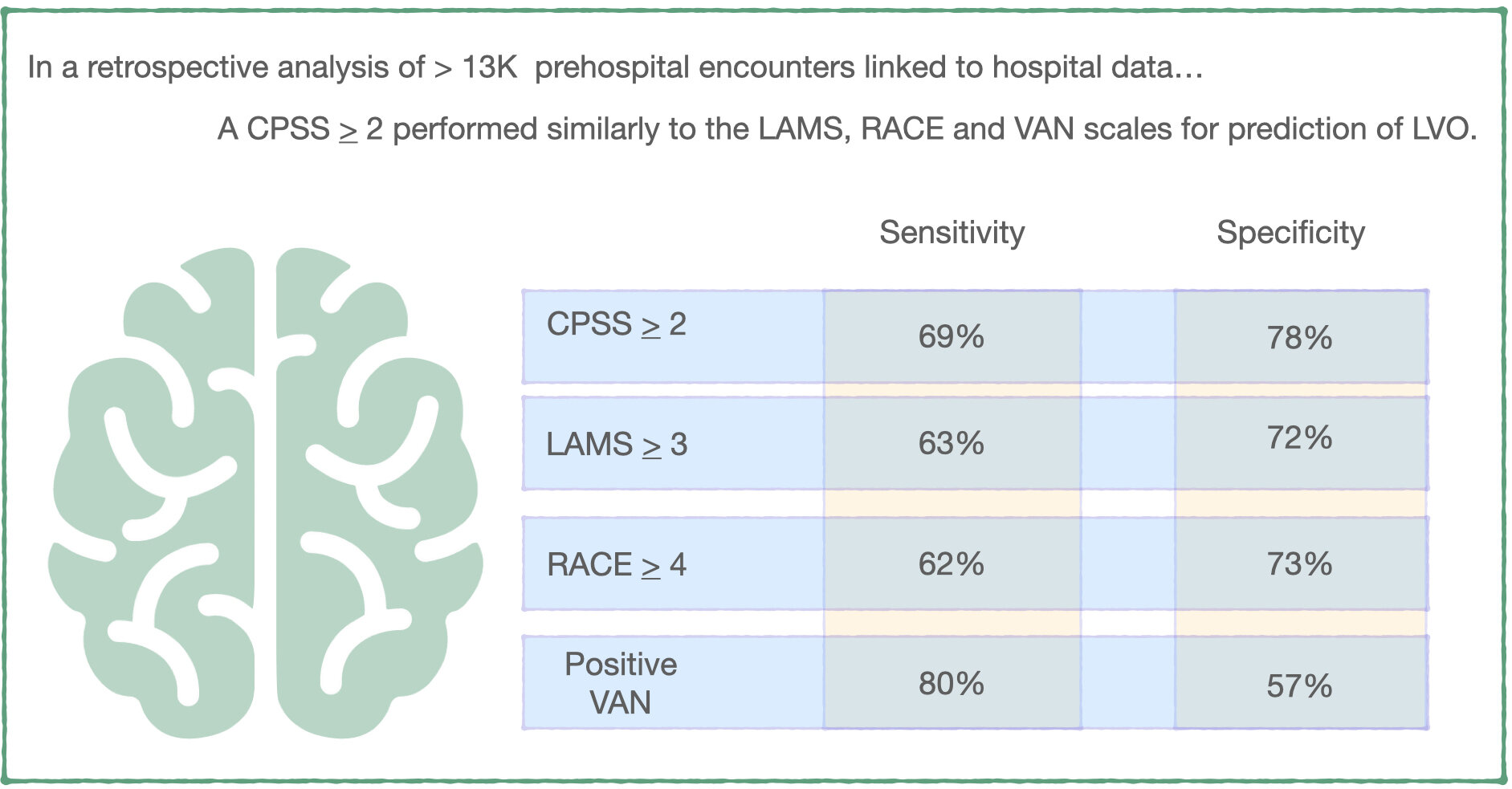
- Among 13,596 responses from 151 EMS agencies that had one or more stroke scales documented, CPSS was the most commonly documented instrumented (83% of patients), followed by RACE (14% of patients), the LAMS (7% of patients), and VAN (4% of patients). 31% of patients with documented prehospital stroke scales had a hospital ICD-10 diagnosis indicative of stroke.
- Breakdown of types of stroke: 57% ischemic stroke, 23% TIA, 13% with TIA, 7% with multiple types. Of patients experiencing ischemic stroke, 26% had an ICD-10 diagnosis indicative of LVO.
- A CPSS score > 2 (positive on 2 or more physical exam elements of facial droop or palsy; arm weakness, drift or drop; and abnormal speech) had a sensitivity of 69% and specificity of 73% for LVO. There was no statistically significant differences between this and the performance of the RACE, LAMS or VAN scales.
- Among patients with a CPSS score > 2, 14% were diagnosed with LVO and 9% were diagnosed with ICH. 39% did not have a stroke diagnosis.
What this means for EMS: Destination decision is a critical component of the prehospital management of stroke and this decision has increased in complexity with the availability of endovascular therapy for patients with large vessel occlusion. On a system scale, optimizing patient and system outcomes requires a balance between over- and under-triage of these patients to specialty centers capable of providing endovascular therapy. In this retrospective analysis of prehospital patients, a CPSS > 2 performed similarly to more complex LVO scales for large vessel occlusion prediction. While we await assessment of additional prehospital stroke scales, it may be more worthwhile to focus on incorporating CPSS > 2 into destination decisions and quality improvement efforts than training EMS clinicians on new stroke scales.
References:
1. Saver, J. L., Goyal, M., Van der Lugt, A. A. D., Menon, B. K., Majoie, C. B., Dippel, D. W., ... & Cardona, P. (2016). Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. Jama, 316(12), 1279-1289.
2. Jayaraman, M. V., Hemendinger, M. L., Baird, G. L., Yaghi, S., Cutting, S., Saad, A., ... & McTaggart, R. A. (2020). Field triage for endovascular stroke therapy: a population-based comparison. Journal of neurointerventional surgery, 12(3), 233-239.
3. Smith, Eric E., et al. "Accuracy of prediction instruments for diagnosing large vessel occlusion in individuals with suspected stroke: a systematic review for the 2018 guidelines for the early management of patients with acute ischemic stroke." Stroke 49.3 (2018): e111-e122.
Article Summary by Maia Dorsett, MD PhD FAEMS, @maiadorsett


















![Figure 4: Sample protocol for non-transport of patients [22]](https://images.squarespace-cdn.com/content/v1/58174dbb6a496367e36143b1/1591378008617-KOZLXLO2K6JAM2ATC0M2/Untitled4.jpg)








