1. Brown JB et al. Not all prehospital time is equal: influence of scene time on mortality.J Trauma Acute Care Surg Jul 2016;81(1):93-100.
Reviewed by Maia Dorsett, MD PhD, @maiadorsett
Background & Objectives: Given that trauma is a time –sensitive disease, minimizing prehospital time may be beneficial to trauma patients. Most studies have not linked increased prehospital time with worse trauma outcomes. With the overall goal of identifying modifiable prehospital factors that may improve trauma outcomes, the objective of the study was to evaluate the association of prehospital time patterns with mortality.
Methods: The study was a retrospective review of data from the Pennsylvania Trauma Registry. Inclusion criteria were all patients 16 years and older who were transported by EMS with a total prehospital time (TPT) of 20 minutes or longer between Jan 2000 – June 2013. Patients were excluded if they were transported from another hospital or if prehospital time data were missing. Total prehospital time was divided into Response (notification to arrival), Scene (arrival to leaving for hospital), and Transport (scene to hospital). In order to control for the variability of “raw” prehospital times due to EMS system characteristics, the authors evaluated the relative proportion that each interval contributed to the TPT. A time interval was classified as prolonged If it contributed to > 50% of TPT.
After matching for TPT, a logistic regression model was used to determine the association of mortality with PH time pattern, controlling for confounders such as age, sex, race, co-morbidities, mechanism, transport mode, PH provider level (ALS vs. BLS), IVF volume, PH and admission VS, ISS, severe head injury, and blood transfusion in the ED.
Key Results: Out of 164,471 patients included in the study, only 2% had a prolonged response time, while 19% had a prolonged scene time and 31% had a prolonged transport time. Prolonged scene time was associated with a 21 % increase in mortality (AOR 1.21, 95% CI, 1.02-1.44, p = 0.03). Prolonged response time and transport time were not associated with increased mortality [AOR 1.16, 95% CI 0.83-1.3, p = 0.38 for response; AOR 0.82, 95% CI 0.65 – 1.04, p = 0.11 for transport]. This was consistent for patients with both blunt and penetrating trauma, although was more pronounced for patients with penetrating trauma who more commonly required emergent operative intervention. The effect was also more pronounced when overall prehospital time increased.
To further break down contributing factors, the authors evaluated the contribution of intubation and extrication to mortality. They found that prehospital intubation was associated with an increased risk of mortality (AOR 4.49, 95% CI, 3.48-5.78) and contributed on average 6 min 22 seconds to scene time. Extrication was also associated with mortality (AOR 1.40, 95% CI 1.19-1.65, p < 0.01) and extended scene time by 4 min 30 seconds on average. Together, they mediated 60.5% of the total effect of prolonged scene time on mortality in the risk-adjusted model. However, the more extrications or intubations an EMS agency performed, the less dramatic the effect on mortality. In patients with GCS < 8, there was an association between prehospital intubation and mortality if patients were transported by ground (AOR 2.52, 95% CI, 1.95 – 3.26, p < 0.01) but not if they were transported by helicopter (AOR, 1.26; 95% CI 0.92 – 1.73).
Take Home: Prolonged scene time is associated with increased mortality of trauma patients. Prehospital intubation and extrication mediate this effect significantly, although less so when EMS agencies have more experience in either procedure.
2. Weaver MD et al. An observational study of shift length, crew familiarity, and occupational injury and illness in emergency medical services workers. Occup Environ Med Nov 2015;72(11):798-804.
Reviewed by Catherine Counts, MHA (@CatherineCounts)
Background& Objectives: EMS is high-risk work where extended shifts and lack of familiarity between teammates is common.
Extended shifts: While OSHA defines a normal work shift as 8 hours a day, 5 days a week with at least 8 hours of rest between shift, EMS providers often work an extended shift which may increase the risk of “adverse events, medical errors and attentional deficits.”
Crew Familiarity: Data from the airline industry has shown that a lack of familiarity between pilots is linked to more errors. The average EMS provider will have 19 different partners annually; some will have as many as 50 in a year and thus lack of familiarity may lead to more errors amongst EMS providers.
Given the high rate of extended shifts in EMS, as well as lack of consistency between EMS partners, the objective of this study was to “examine the relationship between shift length and occupational injury while controlling for relevant shift work and teamwork factors.”
Methods: This was a retrospective study using administrative data from 14 EMS agencies with 37 base sites. Agencies provided historical shift schedules and OSHA reports which were matched by date. The primary outcome of interest was OSHA-reported illness or injury, defined as an injury that required medical treatment beyond basic first aid “or [resulted] in loss of consciousness or an inability to perform normal duties without restriction.” The exposure of interest was shift length; the main analysis stratified continuous shift length variable into sections: less than 8 hours, 8-12 hours, 12-16 hours, 16-24 hours, and 24+ hours. Secondary analysis included shift length as a dichotomous variable (yes/no 12+ hours, yes/no 10+ hours), as well as a continuous variable.
The authors also evaluated the effects of the following independent variables of interest:
· Partner Familiarity - Assigned via number of shifts with partner within past 8 weeks, categorized by quartiles.
· Recovery period – time between end of prior shift and start of shift with injury, treated as continuous variable with 1 hour increments
· Consecutive shift - if less than 2-hour break between shifts
· Overnight shift – Yes/No
· Part time – Work less than 34 hours a week
· Number of workers at agency – estimated using unique number of workers during middle four weeks of study period (workforce size is associated with injury reporting)
Results were analyzed using multi-variable mixed-effects logistic models using both fixed and random effects.
Key Results: Fourteen agencies at 37 sites participated over 1 to 3 year period. OSHA reports were matched on date, location and employee. Shifts were excluded when the assigned job role described a non-clinical task. After removing non-clinical and incomplete shift records, the authors included 966, 082 total work shifts from 4,382 employees in their analysis.
Shifts < 8 hours as well as overnight shifts are associated with fewer injuries [relative risk 0.70, 95% CI 051-0.96, p = 0.029 for < 8 hr; RR 0.78, 95% CI 0.65-0.93, p = 0.005 for overnights. 16-24 hour shifts are associated with more injuries when compared with the reference category of 8-12 hour shifts (RR 1.6, 95% CI 1.22-2.10, p=0.001). Familiarity, agency workforce size, part-time status and hours of recovery were not associated with occupational injury or illness. Consecutive shifts also did not significantly alter the risk of occupational injury or illness. Shifts > 24 hours had nearly a three-fold increase in risk (RR 2.88, 95% CI 1.74 – 4.77, p < 0.001), while shifts over 12 hours had a 38% increase in risk of injury (RR 1.38, 95% CI 1.12-1.70, p = 0.002).
Take Home: There is not a one-size-fits-all model for EMS scheduling, but this study further builds on literature that shift length serves as a contributing factor for employee wellbeing. Before any organizational changes are made “trials of novel, minimally intrusive, intra-shift and inter-shift safety management interventions in the EMS setting are needed.”
3. Berkhemer OA et al; MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med Jan 2015;372(1):11-20.
Reviewed by Maia Dorsett, MD PhD, @maiadorsett
Background & Objectives: Intravenous alteplase (tPA) therapy has a narrow therapeutic window and a large number of contraindications. Moreover, it is less effective at opening proximal occlusions of the major intracranial arteries which account for a third of anterior circulation strokes. Initial trials of endovascular approaches to reperfusion were largely negative, but there were a number of concerns about these trials including lack of imaging to confirm proximal vascular occlusion prior to intervention, long time interval to intervention, and use of earlier versions of mechanical thrombectomy devices.
Given the concerns regarding these early trials, the objective of the MR CLEAN trial was to determine whether “intraarterial treatment plus usual care would be more effective than usual care alone in patients with a proximal arterial occlusion in the anterior cerebral circulation that could be treated intra-arterially within 6 hrs of symptom onset.”
Methods: MR CLEAN was a multi-center randomized clinical trial carried out in the Netherlands. Patients were randomized to treatment-group assignments, underwent open-label treatment, and blinded end-point evaluation.
Inclusion criteria included:
1. > 18 years of age with acute ischemic stroke
2. intracranial occlusion of the distal intracranial carotid artery, middle cerebral artery (M1 or M2), or anterior cerebral artery (A1 or A1) confirmed on CT or MR angiography
3. NIHSS > 2
The treatment consisted of intra-arterial treatment of the occlusion. The method of reperfusion (thrombolytic agent, mechanical thrombectomy, or both) was left to the discretion of the interventionalist. If eligible, patients were treated with alteplase or urokinase prior to intervention.
The primary study outcome was modified Rankin scale at 90 days. Secondary outcomes included NIHSS at 24 hrs and at 5 to 7 days post discharge, acivities of daily living as measured by the Barthel index, and health related quality of life as measured by the EuroQol Group 5 Dimension Self-Report Questionnaire at 90 days.
Key Results: The study included 500 participants, 233 (46.6%) were assigned to the intervention group and 267 patients (53.4%) were assigned to the control group. The large majority of patients received IV alteplase prior to intra-arterial intervention (87.1% in intervention group and 90.6% in control group), with a median time to initiation of 85-87 min. 196 of 233 participants in the treatment group received intra-arterial therapy.
For the primary outcome of modified Rankin score, there was a shift in favor of the intervention with an adjusted common odds ratio of 1.67 (95% CI 1.21-2.30). Patients who received intra-arterial treatment were more likely to be functionally independent (modified Ranken score 0 to 2; AOR 2.16; 95% CI, 1.39-3.38) with an absolute increase of 13.5% (32.6% vs. 19.1%, NNT 7.4). Patients receiving intra-arterial treatment also scored higher on the Barthel index (AOR for score 19 or 20 at 90 days 2.1, 95% CI 1.4 – 3.2). There was no significant difference in QQ-5D score (69% vs. 66%).
There was no significant difference in severe adverse events between the two groups during the 90 day follow-up period. Procedure-related complications included embolization into new vascular territories downstream of the occluded vessel (8.6% of patients) with evidence of new ischemic stroke in a different vascular territory in 5.6% patients in the intervention group.
Take Home: There is a functional benefit of intra-arterial therapy for patients with acute ischemic stroke with NIHSS > 2 and confirmed proximal intra-arterial occlusion in the anterior circulation who are able to receive treatment within 6 hrs.
4. Drennan IR, Lin S, Sidalak DE, Morrison LJ. Survival rates in out-of-hospital cardiac arrest patients transported without prehospital return of spontaneous circulation: an observational cohort study. Resuscitation. 2014;85(11):1488-93.
Reviewed by Jeremiah Escajeda, MD, @ JerEscajeda
Background & Objectives: Cardiac arrest is a prevalent disease entity encountered in EMS systems. In 2009, Morrison et al. derived and validated the prehospital Universal Termination of Resuscitation (TOR) Guideline to help direct appropriate termination efforts for both basic life support (BLS) and advanced life support (ALS) prehospital services. To satisfy the TOR Guideline, a cardiac arrest patient must have an 1) unwitnessed arrest by EMS personnel 2) no shock delivered and 3) no return of spontaneous circulation (ROSC) [1]. Implementation of the TOR has been inconsistent and some prehospital services use sole criteria for termination of resuscitation based on no ROSC achieved. The authors sought to report the survival rates of patients without prehospital ROSC who still met transportation criteria based on the TOR Guideline, such as those still who have had either witnessed arrest by EMS personnel or prehospital shock delivered.
Methods: This was a retrospective observational study of the Toronto site Resuscitation Outcomes Consortium (ROC) database from April 1, 2007 –March 31, 2013. Subjects included for analysis were adult patients (≥18 years of age) with cardiac arrest suspected to be cardiac etiology (no trauma, drowning, overdose or asphyxia). EMS services included both BLS (supraglottic device capable) and ALS services. Both EMS and inhospital records were reviewed. 20,207 patients met inclusion criteria.
Key results: Of the 20,207 adult cardiac arrest patients included, 3,374 (16.4%) did not have prehospital ROSC but met the Universal TOR guideline for continued resuscitation and transport. Of these, 551 (16.3%) obtained ROSC in the ED and 122 (3.6%) survived to hospital discharge.
In adjusted multivariable logistical regression, survival to discharge was associated with younger age (OR 0.98; 95% CI 0.97-0.99), initial shockable VF/VT (OR 5.07; 95% CI 2.77-9.30), EMS witnessed arrests (OR 3.51; 95% CI 1.73-7.15), bystander-witnessed arrests (OR 2.11; 95% CI 1.18-3.77) and public locations (OR 1.57; 95% CI 1.02-2.40).
Take home: In this study, using absence of prehospital ROSC as sole determinant for TOR misses an unacceptably high number of potential survivors (3.6%). This is well above the 1% defined threshold for medical futility. Also, the optimal minimum time to obtain prehospital ROSC has not been established. Adherence to all of the Universal TOR criteria results in more accurate prehospital identification of futility in cardiac arrest patients.
1. Morrison LJ, Verbeek PR, Zhan C, Kiss A, Allan KS. Validation of a universal prehospital termination of resuscitation clinical prediction rule for advanced and basic life support providers. Resuscitation. 2009;80(3):324-8.
5. Nichol G et al.; ROC Investigators. Trial of continuous or interrupted chest compressions during CPR. N Engl J Med Dec 2015;373(23):2203-14.
Reviewed by Tom Grawey, DO @EMtgDO
Background & Objectives: Standard CPR consists of manual chest compressions with positive-pressure ventilation until ROSC is obtained. Traditionally chest compressions are interrupted by ventilation, though interruptions reduce circulation of blood and potentially reduce the effectiveness of CPR. One way to reduce time off the chest in CPR is to provide breaths to the patient while continuing CPR. The objective of this study was to determine the effect of continuous chest compressions at a rate of 100 per minute with ventilation provided concurrently at a rate of 10 per minute (experimental group) compared to interrupted compressions with a 30:2 compression:breath ratio (control group) during CPR on the rate of survival, neurologic function, or the rate of adverse events.
Methods: This was a cluster-randomized trial with crossover; 114 EMS agencies across 8 sites were grouped into 47 clusters were randomly assigned to performed continuous or interrupted compressions to all cardiac arrests to which they responded. Twice yearly the cluster was switched to the other resuscitation strategy. Both protocols were followed for a total of 6 minutes, at which point an advanced airway was placed and all patients underwent continuous compressions with breaths provided at a rate of 10 per minute. CPR quality was measured in both groups. The primary outcome was survival to hospital discharge with secondary outcomes including neurologic function at discharge (using modified rankin scale based on the clinical record), adverse events and hospital-free survival (number of days alive and permanently out of the hospital during the first 30 days after the arrest).
Key Results: 1129 of 12,613 patients (9%) in the continuous compressions group and 1072 of 11035 (9.7%) in the 30:2 group survived to hospital discharge. In patients with available data on neurologic status, 883 of 12,560 patients (7%) in the intervention group and 844 of 10,955 (7.7%) in the control group survived with a modified Rankin scale score of 3 or less. Hospital-free survival was significantly shorter in the intervention group than in the control group (mean difference, -0.2 days; 95% CI, -0.3 to -0.1; P=0.004) however this is arguably not clinically significant.
Take home: The study authors conclude that among patients with out of hospital cardiac arrest, continuous chest compressions did not result in significantly higher rates of survival or favorable neurologic status when compared to a 30:2 compression to ventilation ratio. Despite the differences in CPR protocol, the mean difference in chest compression fraction (defined as the proportion of each minute during which compressions were given) between the intervention and control groups was small (0.83±0.14 and 0.77±0.14 ; p<0.001 respectively) in this study which may be contributing to similar outcomes and may emphasize the importance of minimizing compression interruptions. It is unclear whether ventilation quality and strategy contributed to patient outcomes as it was not measured.
6. Fisher R, et al. Police officers can safely and effectively administer intranasal naloxone. Prehosp Emerg Care 2016;20(6):675-80.
Reviewed by Aurora Lybeck, MD, @AuroraLybeck
Background & Objectives: Law enforcement officers (LEOs) are increasingly utilizing naloxone administration for suspected opioid overdose patients prior to EMS arrival, though limited literature is available on the safety and efficacy of such programs. This publication provides an example of training and implementation of LEO administered naloxone program, and aimed to describe indication, response, and disposition of the patients to whom LEO naloxone was administered.
Methods: This is a retrospective case series describing LEO administered naloxone within a large urban police department and included 126 occurrences over 18 months of data collection. They described a 30-minute training program for all LEOs and academy trainees. Instruction topics included signs and symptoms of suspected opioid overdose, naloxone and atomizer devices with hands-on training and instruction to administer 2mg IN naloxone, and required patient transport to the hospital if LEO naloxone was administered. The data was gathered through standard police run reports and an additional naloxone administration data form, using the narrative to complete data when possible. Data elements included:
1) number of times naloxone was administered by police
2) indications for naloxone administration
3) basic patient demographics
4) patient response to naloxone
Other data elements collected included time to EMS arrival on scene and whether the patient was voluntarily or involuntarily transported to the hospital. If patient refused transport, LEO placed patient on involuntary hold and transported them to hospital.
Key Results: The most common indication for LEO administered naloxone was “unconscious/unresponsive” (n=117, 92.9%). Most patients receiving LEO naloxone were white (92.9%), male (59.5%), with average age of 32.8 years. After LEO administration of naloxone, most patients regained consciousness (n=82, 65.1%) or regained spontaneous respirations (n=71, 56.3%), though some demonstrated no response (n=22, 17.5%). Of those with no response to a single dose of LEO administered naloxone, 18 of the 22 outcomes were reported: 3 were fatalities, 3 were non-opioid overdoses, and 12 responded to an additional dose of naloxone by EMS. EMS arrived within 5 minutes in 90% of the calls. 96.8% were transported to the hospital voluntarily. One patient became agitated but later went voluntarily to the hospital. No significant adverse effects noted.
Limitations of the study included selection based only on LEO naloxone administration, potentially missing those opioid overdoses cases not recognized by the LEO, 28 reports with missing EMS follow-up data, lack of toxicological confirmation tests confirming opioid as primarily intoxicant.
Take Home: Trained law enforcement officers can correctly identify an opioid overdose and effectively administer naloxone without significant adverse effects. Further research is needed regarding outcomes and system impact.
7. Rostykus P et al. Variability in the treatment of prehospital hypoglycemia: a structured review of EMS protocols in the United States. Prehosp Emerg Care 2016;20(4):524-30.
By Hawnwan P. Moy, MD, @PECpodcast
Background & Objectives: Hypoglycemia is a frequent emergency situation in many prehospital systems. The traditional approach has been to treat hypoglycemia intravascularly (IV) with fifty milliliters of a 50% solution of glucose containing 25 grams of glucose (commonly known as an amp of D50). However, this treatment is not without risk. D50 is known to over-treat hypoglycemics to inappropriate hyperglycemic levels. Mathematically speaking an amp of D50 is 5 times the amount of glucose in a normal adult and a common pediatric dose of 0.5-1 g/kg (at more dilute concentrations of D25, D12.5, or D10) of glucose provides 6-11 times the normal amount of glucose in a normal child’s blood. Excessive glucose can cause complications in the brittle diabetic and has been known to have detrimental effects in medical conditions such as acute stroke and post cardiac arrest patients. The hypertonic nature of D50 may also cause tissue necrosis should extravasation occur. Finally, dilution and adjustment for the hypoglycemic pediatric population is highly prone to error.
In response to a national drug shortage of D50 and potential harmful side effects, many EMS systems adjusted their treatment of hypoglycemia to now include a 10% dextrose containing solution (D10) instead of D50. Initial studies on D10 indicate that there is a similar time to reversal of hypoglycemia, less post treatment hyperglycemia, and less risk of tissue necrosis given the lower hypertonicity of D10 compared to D50. As a result, this objective of this study was to determine how many EMS systems utilize D10 in their treatment protocols in place of D50. Secondary outcomes were to describe initial and subsequent dextrose treatments, routes of administration, availability of glucagon to treat hypoglycemia, recommendation for post treatment monitoring, and non-transport policies for treated patients.
Methods: The authors performed a structured review of EMS protocols from 50 of the largest populated cities in the United States as well as EMS protocols from http://www.emsprotocols.org. The following data points were manually abstracted by trained investigators: the concentration of glucose recommended for the parenteral reversal of hypoglycemia in adult and pediatric patients, clinical treatment thresholds, dose recommendations, follow-up care, glucagon use, and non- transport policies.
Key Results: The authors collected a total of 185 protocols. From those protocols, 70% had D50 as the only treatment for hypoglycemia, 8% mandated D10 as the only treatment, and 22% allowed either D50 or D10. For the pediatric population, two thirds of the EMS protocols called for 0.5 g/kg of hypertonic glucose with the rest varying between 1 g/kg to less than 0.5 g/kg. The most common initial dose for neonates was 0.5 g/kg. Additionally, IV and intraosseous (IO) routes of administration were allowed in two-thirds of protocols, and a third allowed only IV. Other major differences included post treatment guidelines (only a third of protocols had this) and less than half of all protocols had a non-transport policy of patients with corrected glycemic status.
Take Home: Despite the fact that the administration of D50 has the known side effects of supratherapeutic blood sugar, a majority of EMS systems still utilize D50 for treatment of hypoglycemia. Additionally, this manuscript demonstrates a wide variability in treatment of hypoglycemia in not only the dosage of medication used, but also blood glucose indication for treatment, subsequent dosage of glucose treatment, potential routes, post treatment monitoring, and non-transport policies for treated patients. In many industries, reducing variabilities in protocols reduces errors. Although no two EMS systems are alike, if there is no physiologic or scientific basis for protocol differences, treatment standardization may result in fewer errors and enhance patient safety.
8. Prekker ME et al. Pediatric Intubation by paramedics in a large emergency medical services system: process, challenges, and outcomes. Ann Emerg Med Jan 2016;67(1):20-9.
Reviewed by Maia Dorsett, MD PhD, @maiadorsett
Background & Objectives: In many EMS systems, pediatric intubation is considered a core paramedic skill. However, proficiency in pediatric intubation is hampered by inadequate training, infrequent opportunities to perform the skill, as well as anatomic and equipment differences. The largest clinical trial of pediatric intubation by paramedics found that pediatric intubation and bag-mask-ventilation had comparable survival and neurologic outcomes while intubation delayed transport to the hospital [1]. Evaluating the process used by paramedics in systems in which pediatric intubation is performed is a starting point for quality improvement.
The study had two objectives:
1. Estimate “the incidence of out-of-hospital pediatric intubation within the study community and the EMS system”.
2. Describe “the process used by paramedics in their attempt to intubate these children, with focus on the specific challenges to successful intubation, the corrective actions taken after a failed intubation attempt, and potential procedural complications.”
Methods: The study is a retrospective cohort study of patients < 13 years old who underwent at least one intubation attempt treated by the EMS system serving King County, WA between September 2006 and December 2012. Paramedics in the King County system receive standardize training in airway management and perform an average of 6 pediatric and 40 adult intubations prior to graduation from paramedic school They are also required to perform 12 successful intubations per year.
Data collection involved retrospective chart review of EMS, ED and hospital reports as well as review of a mandated detailed airway report that paramedics must complete for each attempted intubation.
Key Results: In 651,194 calls over 6.3 years, only 299 encounters included an attempted pediatric intubation (0.05% of all calls). Among the 123 paramedics who had at least one pediatric intubation attempt, on average paramedics had no more than one pediatric intubation every 2.6 years. Cardiac arrest was the most common reason(44%) and most commonly involved infants. Preschool and school age children were most commonly intubated for neurologic emergencies, including seizures and trauma.
Amongst all intubation attempts, first pass success was only 66%. First pass success was lower in infants (53%) and children in cardiac arrest (53%). Second attempt success rate was 69% (cumulative success after two attempts was 89%), 57% on 3rd attempt (cumulative success 95%) and 64% after > 4 attempts (cumulative success 97%). The most commonly noted challenge to successful intubation was body fluids (33%). Paramedics changed their approach after failed intubation by suctioning the airway (32%), repositioning the patient (27%), or changing the operator or using a bougie after a failed third attempt. Among the 8 patients who were not successfully intubated out-of-hospital, 5 were intubated in the ED, 1 died prior to arrival and 2 did not end up requiring intubation. Of note, 9% of pediatric patients who were intubated out-of-hospital were extubated in the ED and 1% were discharged home.
The most frequent complication was right mainstem intubation (19%). Major complications (peri-intubation arrest, ET tube dislodgement, injury to the respiratory tract, or bradycardia) occurred in 11% of cases. There were no un-recognized esophageal intubations documented, but endotracheal tube placement could not be retrospectively determined in 50 cases in which the resuscitation was terminated in the field.
Take Home: Pediatric intubation is rarely performed. Even in a system where it is considered a core skill, repeated attempts at intubation were required in a third of patients, although 97% were successfully intubated eventually. Evaluating the process and complications step by step will help determine whether out-of-hospital pediatric intubation is appropriate for an EMS system and opportunities for improvement.
1. Gausche, M., Lewis, R. J., Stratton, S. J., Haynes, B. E., Gunter, C. S., Goodrich, S. M., ... & Seidel, J. S. (2000). Effect of out-of-hospital pediatric endotracheal intubation on survival and neurological outcome: a controlled clinical trial. Jama, 283(6), 783-790.
9. Schlapbach LJ et al. High-flow nasal cannula (HFNC) support in interhospital transport of critically ill children Intensive Care Med Apr 2014;40(4):592-9.
Reviewed by Maia Dorsett, MD PhD, @maiadorsett
Background & Objectives: Escalating respiratory support during transport is inherently difficult; interhospital transport teams have therefore historically had a low threshold for intubating patients prior to transport. However, intubation and invasive ventilation are not without risk. In Pediatric Intensive care, high-flow nasal cannula (HFNC) therapy is increasingly used for respiratory support in critically ill infants and has reduced the need for intubation. The use of HFNC during interhospital transport had not yet been studied. The objective of this study was to evaluate the safety of HFNC during interhospital transfer of critically ill children < 2 years of age and determine the impact of its use on intubation rate and successive PICU management.
Methods: This was a single center, retrospective, pre-post study of critically ill children under 2 years of age requiring interhospital transport by a specialized tertiary pediatric retrieval team and consecutively admitted to the PICU of Mater Children’s Hospital in Brisbane, Australia between 1/2005 and 12/2012. The authors compared the use of respiratory support modes [low flow oxygen/room air, HFNC, NIV (CPAP/BiPAP), or invasive ventilation] and complication rates [need for intubation, pneumothorax, cardiac arrest, need for invasive ventilation in the first 24 hrs of PICU admission] during the 48 month period before and after HFNC was made available as a standard treatment for interhospital transport.
Key Results: 331 children were transported in the pre-HFNC and 462 in the post-HFNC period, with a mean duration of transport of 1.4 hours. After introduction of HFNC, 33% of all patients were transported on HFNC. Intubation rates decreased between the pre (49%) and post(35%) periods. This was in part due to decrease rate of intubation by the referring hospital (36% pre/28% post), but fewer patients were intubated by the retrieval team as well (13% pre/7% post).
In the subgroup of patients with bronchiolitis, there was a significant decrease in the proportion of patients with invasive ventilation initiated by the retrieval team (33% vs. 15%, p = 0.001). Among patients receiving HFNC, bronchiolitis was the most common condition requiring transport (77%).
There was no increase in adverse events post introduction of HFNC, including no cases of patients requiring intubation during transport. There was no significant difference between need for intubation during the first 24 hrs of PICU admission in the pre/post period.
Take Home: HFNC therapy during transport of critically ill infants appears to be safe and may have the potential to reduce need for intubation and invasive ventilation.
10. Clemency BM, Bart JA, Malhotra A, Klun T, Campanella V, Lindstrom H. Patients immobilized with a long spine board rarely have unstable thoracolumbar injuries. Prehosp Emerg Care 2016;20(2):266-72.
Reviewed by Brandon Bleess, MD @BBBleess
Background & Objectives: Because of concern that patients with unstable spine injuries are at risk for secondary mechanical injury if exposed to significant movement, long spine boards and cervical collars were widely adopted as a mainstay of treatment for patients with suspected spine injury. However, multiple studies have demonstrated potential harms of spine board use, including respiratory compromise, pain, tissue ischemia, and unnecessary imaging. In order to objectively compare the relative risk and benefit of spinal immobilization, the objective of this research was to “determine the prevalence of unstable thoracolumbar spine injuries among patients receiving prehospital spine immobilization.”
Methods: This was a retrospective study of prehospital and hospital records of patients cared for by a single, large, private EMS agency in Western New York from January 1, 2010 through December 31, 2013. The corresponding hospital data was obtained from a single, Urban, academic Level I Trauma Center that served an 8 county region with approximately 65,000 emergency department visits per year. During the study, EMS operated under a single statewide “Suspected Spinal Injury” protocol requiring complete spinal immobilization when spinal injury was suspected. Inclusion criteria included age > 18 years old, documented spinal immobilization from the scene, and transport to the study hospital.
Hospital records were reviewed for mechanism of injury, imaging of the thoracolumbar spine, and presence of any acute fractures, dislocations, or subluxations. For patients with injuries identified on spinal imaging, performance of thoracolumbar spine surgery during hospitalization was used as a marker for unstable thoracolumbar spine injury. The primary outcome was the percentage of patients with unstable thoracolumbar injury who underwent prehospital spine immobilization following blunt trauma. Rate of imaging and injuries by mechanism were secondary outcomes.
Key Results: Immobilization was documented on 5,593 patients transported to the ED. 97% of prehospital records were successfully linked with the corresponding hospital record. Spinal imaging was ordered in 82.5% of subjects and thoracolumbar imaging specifically was ordered in 51.3%of subjects. An acute thoracolumbar fracture, dislocation, or subluxation was present in 4.3% of cases. An unstable injury was present in 0.5% cases (n=29). No unstable injuries were found among the 951 patients that were immobilized following ground level falls. Falls from heights greater than 20 feet had the greatest chance of causing any fractures and unstable injuries with 10% (8/80) having unstable fractures.
Take home: While spinal imaging is commonly performed on blunt trauma patients who undergo prehospital spinal immobilization, unstable thoracolumbar injury is a rare occurrence.
11. Newgard CD et al.; Western Emergency Services Translational Research Network (WESTRN) Investigators. Improving early identification of the high-risk elderly trauma patient by emergency medical services. Injury Jan 2016;47(1):19-25.
Reviewed by Melody Glenn, MD, @MGlennEM
Background & Objectives: Existing field triage guidelines often fail to identify serious injuries in elderly trauma patients. This is partially due to the increased likelihood of sustaining injury from low-velocity mechanisms (e.g. ground-level falls) and their different physiologic responses to injury amongst the elderly. Under-triage leads to a greater proportion of seriously injured elders being transported to non-trauma hospitals, where their needs may outstretch hospital capabilities. Although the CDC added a “special consideration” section relating to adults >55 into their 2011 field triage guidelines for injured patients, little evidence exists that similar EMS protocol changes have decreased under-triage.
The objectives of the study were to:
- Define the high-risk injured older adult using prognostic differences associated with different injury patterns
- Derive alternative field trauma triage guidelines that mesh with current national guidelines to improve identification of high-risk elderly trauma patients.
Methods: This was a retrospective cohort study of 33,298 injured adults 65 years or older who were transported by 94 EMS agencies to 122 hospitals in 7 western regions in the United States from 2006 - 2008. Only patients with matched hospital records were included. The researchers used Abbreviated Injury Scale (AIS) scores or need for surgery to create 5 definitions/categories of “serious injury,” including: Injury Severity Score (ISS) ≥ 16, serious traumatic brain injury (TBI), serious chest injury, serious chest injury, serious abdomen-pelvic injury, and serious extremity injury. They considered in-hospital mortality as a marker of prognosis to compare definitions.
They derived an alternative set of field triage guidelines to identify high-risk older adults, using 60% of the sample to derive and cross-validate their decision tree. The remaining 40% were used to validate the tree.
Key Results: 80% of the elders in their sample of 32,298 patients were injured by falls. Out of their cohort, 13,401 met their definition for serious traumatic injury (4.5% with ISS ≥ 16, 4.7% with TBI, 3.4% w serious chest injury, 1.7% with Abdomen-Pelvis injury, and 3.1% with in-hospital mortality). Patients with isolated serious extremity injuries had the lowest mortality.
Based on having any positive triage criterion from the 23 field trauma triage criteria currently in existence at the study sites, 3,299 serious trauma patients would have been identified, resulting in a sensitivity of 75.9% (95% CI 72.3-79.2%) and a specificity of 77.8% (95% CI 77.1-78.5%).
Their alternative triage guidelines included: any positive triage criterion from the current guidelines, GCS ≤ 14, and abnormal vital signs. Adding these triage criteria resulted in identifying an additional 4,744 patients with serious traumatic injuries, yielding a higher sensitivity of 92.1% (95% CI 89.6-94.1%) and a lower specificity of 41.5% (95% CI 40.6-42.4%).
Take Home: Elderly-specific triage guidelines can be applied to the current national triage guidelines, and will result in greater identification of those with serious traumatic injuries. However, such changes would also result in over-triage.
Further studies should evaluate additional variables/assessment tools that could be used to increase sensitivity without decreasing specificity, the cost and resource implications of adopting new, less-specific triage guidelines, and whether or not there is a survival benefit associated with the treatment of elderly trauma patients at major trauma centers.
12. Scerbo MH, Mumm JP, Gates K, Love JD, Wade CE, Holcomb JB, Cotton BA. Safety and Appropriateness of Tourniquets in 105 Civilians. Prehosp Emerg Care. 2016 Nov-Dec;20(6):712-722.
Reviewed by Scott Goldberg, MD MPH, @EMS_Boston
Background & Objectives: Tourniquets have been commonly used in the military environment for some time. However, tourniquet use in the civilian setting is still controversial and wide variations exist in recommendations for tourniquet use in the civilian population. The objective of this study was to examine tourniquet application in the civilian setting as well as evaluate the safety and efficacy of application by prehospital and emergency department (ED) providers.
Methods: This was a single center retrospective cohort study including all trauma activations from October 2008 through May 2013 in which a tourniquet was applied in the field or in the ED. All tourniquets used in this system were Combat Application Tourniquets (CAT). The authors defined tourniquet use as absolutely indicated if it met any of the following criteria: urgent or operative intervention for limb injury within 2 hrs of arrival or a vascular injury requiring repair or ligation. Tourniquet use was considered relatively indicated if there was documentation of significant blood loss at the scene or a major musculoskeletal/soft tissue injury requiring a non-emergent or urgent operation (between 2 and 8 hours of hospital arrival).
Key Results: Over the study period there were 105 tourniquet applications included for analysis, of which 14 were placed in the ED with the remainder placed in the field. Nine patients had a tourniquet placed in the field and an additional tourniquet placed after arrival to the ED. Approximately an equal number of tourniquets were placed for penetrating and blunt trauma.
Ninety percent of tourniquet placements met the apriori definition of indicated for placement. All of the 10 non-indicated tourniquets were placed in the field. However, it is important to differentiate not indicated from inappropriate. All of the tourniquet applications in this cohort were placed with the intent to control major hemorrhage, and while on final determination of the patient’s injury the tourniquet may have been deemed not indicated, its use was nevertheless felt to be appropriate by the field provider.
There were no significant differences in patient ages, transport times, injury severity scores, or vital signs between indicated and non-indicated tourniquet placement. Thirty percent of patients underwent limb amputation, none of which were related to tourniquet use. Eighteen percent of patients had a complication potentially related to tourniquet use, including amputation, renal failure, compartment syndrome, nerve palsy, or venous thromboembolism. However, after review of each of these cases, none of the complications were felt to be related to use of the tourniquet, with the majority of complications resulting from the injury itself.
Take Home: Tourniquet application was safe and effective in this civilian patient population. The complications seen in the cohort were related to the injuries sustained and not attributable to tourniquet use. Tourniquets should therefore be considered as a potentially life-saving measure for patients suffering from hemorrhage from blunt and penetrating trauma.
Prefer a pdf version? Download one here.












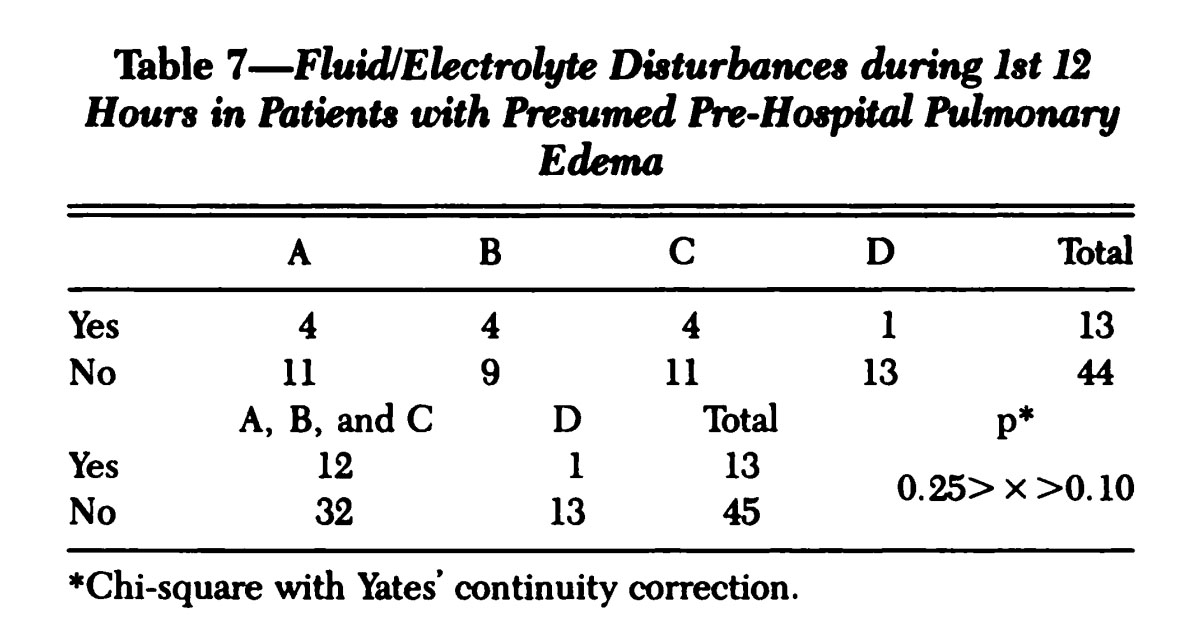
![Pan et. al. Table 3 [Reference 7]](https://images.squarespace-cdn.com/content/v1/58174dbb6a496367e36143b1/1505327105970-YJPOUOUJET70KQTL4IBT/Pan_Table3.jpg)